What if I told you carbon monoxide detectors are a scam? Well I'd be lying. Carbon monoxide is a poisonous gas that gets into your red blood cells and messes everything up; knowing when it's in your house is worth the investment. It doesn't help that it's colorless and odorless either. We had some furnace troubles last winter and after a night of not being sure if we'd die quietly in our sleep, I decided to buy a CO detector ($20 at my local hardware store). In order to understand why carbon monoxide is so deadly, we first need to learn a little about how the body transports oxygen.
It starts with red blood cells. These are special cells that are really rich in a molecule called hemoglobin. It's a big curled up protein with an iron atom in just the right place. This iron atom wants electrons and holds on to the extra ones that are in a molecule of oxygen. The rest of the protein makes sure there's a space that's just big enough to fit an oxygen molecule (more on this later). Each molecule of hemoglobin has four of these iron centers. When the red blood cells are in a place with lots of oxygen, like your lungs, these four sites fill up quickly. When the cells are in an area with low oxygen, like your muscles during a workout, then hemoglobin rearranges and lets go of the oxygen so your muscle can use it to turn carbs into energy.
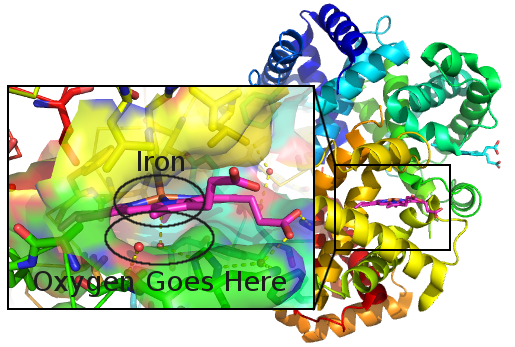
An iron atom can bind an oxygen molecule while the rest of the protein makes a nice pocket that keeps other things out of the way.
